it's ethically tricky, but there are ways to get it right
- Written by Xavier Symons, Postdoctoral Research Fellow, Australian Catholic University
The much anticipated rollout of the Pfizer-BioNTech COVID-19 vaccine will begin in Australia on Monday.
The first groups to receive the jab will be quarantine and border workers, frontline health-care workers, aged-care and disability-care workers, and aged-care and disability-care residents.
For aged-care residents, their age, health and living situation makes them especially susceptible to becoming very sick or dying from COVID-19. So it’s right they are receiving priority access to a vaccine.
But there are also ethical issues that arise when administering vaccines to aged-care residents, who often have diminished capacity to provide consent. Health authorities now face a significant challenge to ensure older members of the community feel safe, comfortable and respected during the vaccination process.
A vulnerable group
One challenge of vaccination in aged care is the fact many older people have dementia, or other conditions that affect their ability to communicate and process information.
Around one in 15 Australians over the age of 65 have dementia, and the risk of developing some form of dementia increases significantly once people enter their 70s and 80s. Among a host of challenges, dementia makes it more difficult for people to consent to medical treatment.
Older people with dementia may also become upset and agitated when things change in their routine or living environment. This means they can easily become distressed during medical procedures.
Read more: Why we should prioritise older people when we get a COVID vaccine
Evidence supporting the safety of COVID-19 vaccines is growing every day. We do know, however, that mild side-effects like headache, fever and chills are more common in COVID mRNA vaccines than standard flu vaccines (the Pfizer vaccine is based on mRNA technology).
These mild side-effects may be exacerbated when someone is already frail and suffering from several pre-existing illnesses.
That said, trial data suggests people over the age of 55 are less likely to experience side-effects from the Pfizer vaccine than younger people.
 Health-care workers will provide information to aged-care residents about the vaccine.
Shutterstock
Health-care workers will provide information to aged-care residents about the vaccine.
Shutterstock
Informed consent
A special team of health-care workers assembled by the federal government will administer COVID vaccines in aged care. These health-care workers must obtain consent from residents receiving the vaccine.
One challenge here is determining whether aged-care residents have capacity to consent. Capacity refers to a person’s ability to make their own decisions.
Generally, a person is said to have capacity if they understand the information relevant to the decision, and the effect of the decision. In the case of vaccination for COVID-19, people must understand they are receiving a vaccine for coronavirus. They must also be made aware of relevant risks and benefits of the vaccine.
The concept becomes more complicated when a patient has a condition like dementia, as their decision-making capacity can ebb and flow depending on the time of day, their location, and the support they have when receiving information.
Read more: Social housing, aged care and Black Americans: how coronavirus affects already disadvantaged groups
Unfortunately, the stigma surrounding ageing and physical and cognitive decline means older patients are sometimes subject to prejudice and inappropriate treatment.
It’s important clinicians avoid making assumptions about older patients’ decision-making capacity before speaking to them, and then provide information in a manner the person can understand.
When health-care workers determine a person doesn’t have capacity to consent, they will require what’s called a substitute decision-maker. This is usually someone who has a close and continuing relationship with the person (such as a partner or other family member).
Many people, particularly in aged-care settings, would have completed the relevant legal documentation to appoint a substitute decision-maker (sometimes known as medical power of attorney). Where a substitute decision-maker has not been appointed, aged-care staff must determine who is legally allowed to make decisions on behalf of the patient.
What can we learn from other countries?
Many countries are already weeks or months into their vaccine rollout, so we can take their experiences into account.
One challenge that’s arisen overseas has been tracking down substitute decision-makers when they’re needed. This process can sometimes take days or weeks.
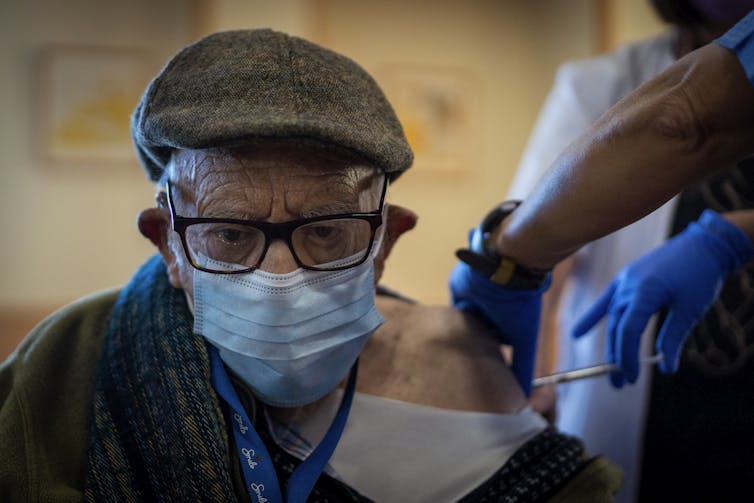 Other countries have already started rolling out COVID vaccines to older people.
Emilio Morenatti/AP
Other countries have already started rolling out COVID vaccines to older people.
Emilio Morenatti/AP
We should also be prepared for complex situations where substitute decision-makers refuse vaccination for those in their care. In a recent case in the United Kingdom, the British Court of Protection ruled it was in the best interests of an 80-year-old woman with dementia and diabetes living in a care home to have the COVID vaccine, despite her son’s objections.
Similar situations will likely come up in Australia. Aged-care staff should contact substitute decision-makers as soon as possible to avoid unnecessary conflicts.
Some aged-care providers have already released messages to residents and their families addressing common concerns about vaccination in general, and COVID-19 vaccines in particular.
Looking ahead
There will be immense pressure on medical practitioners to deliver COVID-19 vaccines quickly to those who are most vulnerable to infection and illness. It already takes significant time and resources to deliver vaccines in aged-care homes, and there may be a temptation to give less importance to consent procedures.
But it’s vital COVID-19 vaccines are given in a manner that respects the autonomy and dignity of older members of the community.
This is particularly important in light of the disastrous response to COVID-19 outbreaks in aged-care facilities during the height of the pandemic in Australia and around the world. Residents’ dignity and autonomy has already been violated once, and we can and should avoid a repeat.
Authors: Xavier Symons, Postdoctoral Research Fellow, Australian Catholic University





