First participant treated at the highest dose level in Clarity's theranostic prostate cancer trial

|
Highlights
- First participant of cohort 3 in the theranostic SECuRE trial investigating 64Cu/67Cu SAR-bisPSMA in metastatic castrate-resistant prostate cancer (mCRPC) has been treated at the highest dose level of 12GBq.
- Cohort 2 was recently completed in 3 participants who received therapy with 67Cu SAR-bisPSMA at the dose level of 8GBq.
- Data from cohort 2 indicates positive effects of the 8GBq dose of 67Cu SAR-bisPSMA on all participants, demonstrated by a reduction in Prostate Specific Antigen (PSA) levels of greater than 50% in all participants within weeks of a single dose.
- No dose limiting toxicities (DLTs) have been reported in any of the trial participants to date.
- Recruitment is ongoing at clinical sites in the US at the cohort 3 dose level of 12GBq of 67Cu SAR-bisPSMA, the highest dose level in the dose escalation phase.
SYDNEY, Aug. 25, 2023 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity"), a clinical stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, is pleased to announce the dosing of the first participant at the highest dose level of 12GBq in the third cohort of its Phase I/II theranostic trial evaluating 67Cu SAR-bisPSMA in participants with metastatic, castrate-resistant prostate cancer (mCRPC).
The SECuRE trial (NCT04868604)[1] is a Phase I/IIa theranostic trial for identification and treatment of participants with Prostate-Specific Membrane Antigen (PSMA)-expressing mCRPC using 64Cu/67Cu SAR-bisPSMA. 64Cu SAR-bisPSMA is used to visualise PSMA-expressing lesions and select candidates for subsequent 67Cu SAR-bisPSMA therapy. The trial is a multi-centre, single arm, dose escalation trial with a cohort expansion involving up to 44 participants in the US. The overall aim of the trial is to determine the safety and efficacy of 67Cu SAR-bisPSMA for the treatment of prostate cancer.
Cohort 3 explores the effects of the highest dose of 12GBq on the SECuRE trial participants following a single administration of 67Cu SAR-bisPSMA. The third cohort will be the last to assess single doses of 67Cu SAR-bisPSMA and will be followed by a multi-dose cohort, pending safety evaluation.
The first two cohorts in the dose escalation phase of the trial were successfully completed with no DLTs reported in any of the participants dosed. The 3 participants in cohort 2, who were administered a single dose of 8GBq of 67Cu SAR-bisPSMA, have been monitored by their physicians for safety and treatment response as per the trial protocol. All 3 participants in cohort 2 remain on the trial following their administration of 8GBq of 67Cu SAR-bisPSMA, with all 3 participants exhibiting a greater than 50% reduction in PSA, which is one of the primary endpoints of the SECuRE trial and a commonly used surrogate endpoint for efficacy in this patient population. PSA levels continue to fall in all patients, with the first 2 participants showing reductions of greater than 95% and the last participant showing a drop of approximately 70% so far.
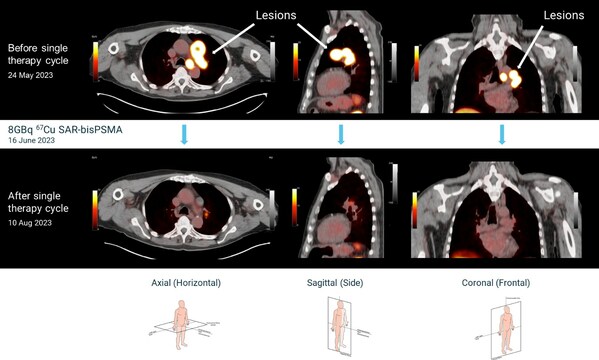 Cu-64 SAR-bisPSMA PET/CT imaging before and after a single cycle of 8GBq Cu-67 SAR-bisPSMA (cohort 2)
Cu-64 SAR-bisPSMA PET/CT imaging before and after a single cycle of 8GBq Cu-67 SAR-bisPSMA (cohort 2)
Clarity's Executive Chairperson, Dr Alan Taylor, commented, "Results from cohort 2 are incredibly exciting and we look forward to seeing data from the increased dosing of 12GBq as well as discover the potential positive effects of multi-dosing on prostate cancer patients.
"The fast pace of recruitment into the dose escalation phase of the trial is indicative of the high unmet need in the prostate cancer therapy space and we are thrilled to be working on a solution that has potential to not only offer treatment benefits to patients with mCRPC, but also resolve the logistical and manufacturing challenges of the current-generation radiopharmaceuticals, such as 177Lu PSMA-617, especially now with the availability of commercial quantities of the 67Cu radioisotope routinely produced domestically in the US and exclusively supplied to us by NorthStar.
"We look forward to sharing more data on 67Cu SAR-bisPSMA as we continue to recruit participants into the SECuRE trial and progress on our path towards commercialisation with the ultimate goal of improving treatment outcomes for people with cancer," said Dr Taylor.
About SAR-bisPSMA
SAR-bisPSMA derives its name from the word "bis", which reflects a novel approach of connecting two PSMA-targeting agents to Clarity's proprietary sarcophagine (SAR) technology that securely holds copper isotopes inside a cage-like structure, called a chelator. Unlike other commercially available chelators, the SAR technology prevents copper leakage into the body. SAR-bisPSMA is a TCT that can be used with isotopes of copper-64 (Cu-64 or 64Cu) for imaging and copper-67 (Cu-67 or 67Cu) for therapy. For more information, see: http://y2u.be/QJz7dyxdEJo.
64Cu SAR-bisPSMA and 67Cu SAR-bisPSMA are unregistered products. Individual results may not represent the overall safety and efficacy of the products. The data outlined in this announcement has not been assessed by health authorities such as the US Food and Drug Administration (FDA). A clinical development program is currently underway to assess the efficacy and safety of these products. There is no guarantee that these products will become commercially available.
About Prostate Cancer
Prostate cancer is the second most common cancer diagnosed in men globally and the fifth leading cause of cancer death worldwide[2]. The American Cancer Institute estimates in 2023 there will be 288,300 new cases of prostate cancer in the US and around 34,700 deaths from the disease[3].
About Clarity Pharmaceuticals
Clarity is a clinical stage radiopharmaceutical company focused on the treatment of serious disease. The Company is a leader in innovative radiopharmaceuticals, developing targeted copper theranostics based on its SAR Technology Platform for the treatment of cancer in children and adults.
www.claritypharmaceuticals.com
References
- ClinicalTrials.gov Identifier: NCT04868604, https://clinicaltrials.gov/ct2/show/NCT04868604
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries, https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21660
- American Cancer Society: Key Statistics for Prostate Cancer, https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html
For more information, please contact: |
|
Clarity Pharmaceuticals |
|
Dr Alan Taylor |
Catherine Strong |
Executive Chairman |
Investor/Media Relations |
+61 406 759 268 |
|
This announcement has been authorised for release by the Executive Chairman.
Authors: PR Newswire
Read more https://www.prnasia.com/story/archive/4192916_CN92916_0




