COVID tests have made pathology companies big profits, but rapid tests are set to shake up the market
- Written by Bruce Baer Arnold, Associate Professor, School of Law, University of Canberra
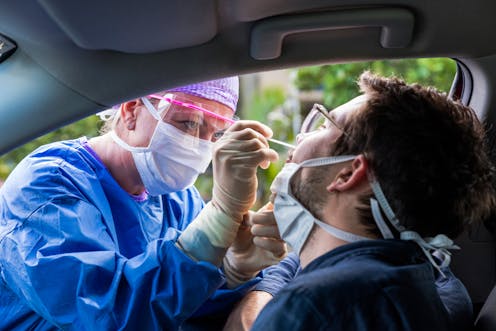
Throughout the pandemic, Australians with a sniffle or other cold and flu symptoms have been encouraged to get a PCR test – a swab of their throat and the back of their nose, taken by a nurse or doctor. This is then sent to a pathology laboratory for analysis.
So far, a total of 41.5 million COVID PCR tests have been performed in Australia. Private companies that process the tests are paid a Medicare rebate of A$85 per test, up from A$28.65 at the start of the pandemic in March 2020.
Although pathology companies undoubtedly have high overhead costs, the sector has recorded massive profits as a consequence of COVID.
But with rapid antigen tests, which can be performed in the home without needing to be sent to a lab, pathology companies could see a drop-off in revenue.
Read more: Home rapid antigen testing is on its way. But we need to make sure everyone has access
Who are the big players and how much profit did they make?
Clinics and practitioners are generally aligned with a particular service provider: consumers don’t get to choose the particular provider.
The pathology sector involves a handful of very large commercial groups operating nationally and often using multiple brand names as a result of takeovers.
The sector also includes much smaller commercial competitors and public sector operations within hospitals.
The large commercial groups typically require significant investment in infrastructure and specialist staff, alongside networks for collecting specimens for testing and communicating results to whoever ordered the test.
There are potential efficiencies in scale: large groups might have more expertise, more capacity and better logistics. The profits are also likely to be higher.
In the 2020-21 financial year, one of the pathology giants, Sonic Healthcare, reported a net profit growth of 149%, to A$1.3 billion, with a significant proportion of that from COVID testing.
The other market leader, Healius, today reported a 44% growth in revenue in the September quarter, compared to 2020, driven by COVID testing and stronger than expected routine testing.
How rapid testing could shake up the system
The pathology industry’s dominance is likely to be eroded in coming decades by the emergence of rapid-testing “diagnostics on a stick” for a range of different illnesses.
These highly specialised low-cost and often disposable (use once and throw away) tools can be used in the home, GP clinic, or even venues such as pubs, clubs, restaurants and universities.
Rapid tests are similar to roadside alcohol testing or off-the-shelf pregnancy tests.
Read more: Rapid antigen tests have long been used overseas to detect COVID. Here's what Australia can learn
For COVID, rapid antigen tests take around 15 minutes and cost about A$8.50 to A$15, but they don’t currently attract a Medicare rebate.
Rapid tests aren’t as accurate as PCR tests, which use high-end equipment and expertise in pathology labs, and are likely to miss some COVID cases. So rapid tests won’t replace PCR tests altogether but are likely to have an important role in testing people without symptoms.
Read more: Rapid antigen testing isn't perfect. But it could be a useful part of Australia's COVID response
Why the delay?
The Therapeutic Goods Administration (TGA) regulates medical devices, ranging from plastic gloves through to pacemakers, and is responsible for authorising new test equipment.
The TGA has been slow to authorise rapid testing tools for COVID. TGA head John Skerritt told News.com the regulator had been waiting for a signal from government about when it was an appropriate time to add rapid antigen tests into the mix.
That signal came in September, with federal health minister Greg Hunt saying he wanted rapid tests to be made available as soon as the regulator had given them the all-clear.
The TGA is now assessing applications from manufacturers for rapid tests that can be used in the home, with the minister expecting these to become available from November.
Slowness is, however, likely to please the big pathology groups, with their eyes on the bottom line and a sense that their world is going to change with advances in technology.
It’s also likely to please the Royal College of Pathologists of Australasia, the professional body representing pathologists.
It has cautioned about undue reliance on rapid tests. It has noted potential issues about accuracy, the handling of devices, and the need to capture data as the basis for effective public health responses when infection spreads across locations.
The government has said rapid antigen tests will play a role in Australia’s COVID response going forward, but we’re yet to see exactly how this will work, who will have access, and at what cost.
But it’s clear we need more transparency about how these decisions are made.
Authors: Bruce Baer Arnold, Associate Professor, School of Law, University of Canberra





