is light a wave or a particle?
- Written by Sam Baron, Associate professor, Australian Catholic University
Is light a wave or particle? — Ishan, age 15, Dubai
Hi Ishan! Thanks for your great question.
Light can be described both as a wave and as a particle. There are two experiments in particular that have revealed the dual nature of light.
When we’re thinking of light as being made of of particles, these particles are called “photons”. Photons have no mass, and each one carries a specific amount of energy. Meanwhile, when we think about light propagating as waves, these are waves of electromagnetic radiation. Other examples of electromagnetic radiation include X-rays and ultraviolet radiation.
It’s worth remembering light — regardless of whether it’s behaving like a wave or particles — will always travel at roughly 300,000 kilometres per second. The speed of light as it travels through space (or another vacuum) is the fastest phenomenon in the universe, as far as we know.
The double-slit experiment
Imagine you have a bucket of tennis balls. Two metres in front of you is a solid panel with two holes in it. A metre behind that panel is a wall. You dip each ball in red paint and throw it at one hole, and then the other. A successful throw will leave a red mark on the wall behind, leaving a specific pattern of roundish dots.
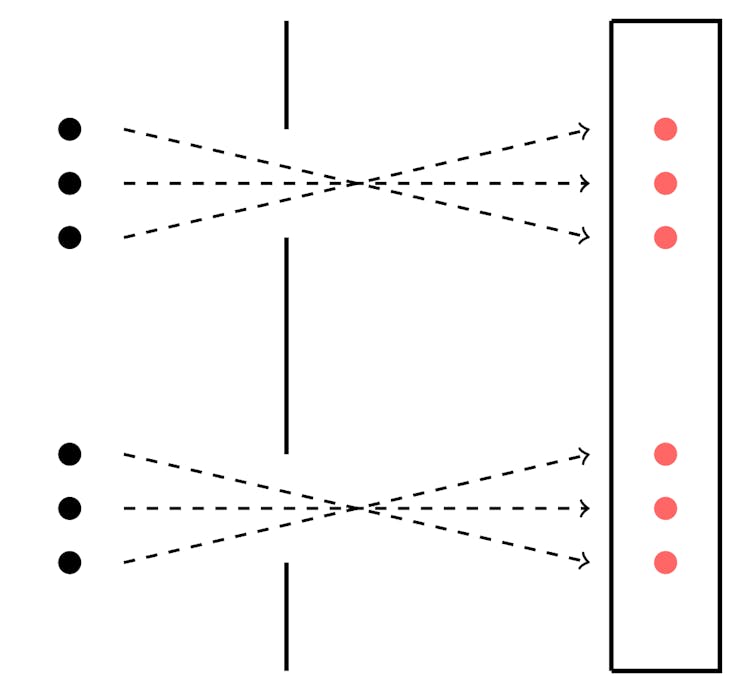 Throw balls at a wall and, if your aim is good, you’ll get a pattern of dots.
Provided by author
Throw balls at a wall and, if your aim is good, you’ll get a pattern of dots.
Provided by author
Now, suppose you shoot a single beam of light at the same panel with holes in it, on the same trajectory as the tennis balls. If light is a beam of particles, or in other words a beam of photons, you would expect to see a similar pattern to that made by the tennis balls where the light particles strike the wall.
That, however, isn’t what you see. Instead, you see a complex pattern of stripes. Why?
This is because light, in this situation, acts like a wave. When we shoot a beam of light through the holes, it breaks into two beams. The two resulting waves then interfere with each other to become either stronger (constructive interference) or weaker (destructive interference).
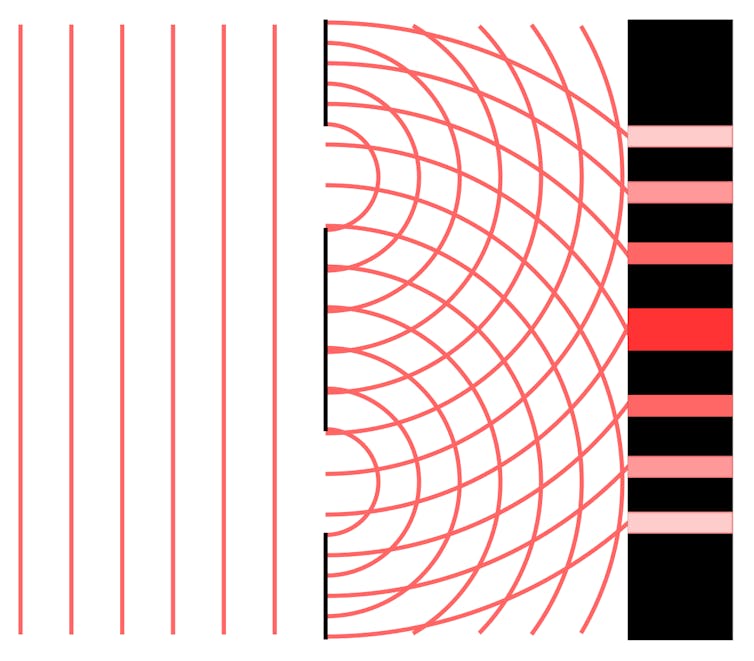 A single wave of light breaks into two, generating what’s called an ‘interference pattern’.
Provided by author
A single wave of light breaks into two, generating what’s called an ‘interference pattern’.
Provided by author
The waves create a lattice pattern, which results in a series of stripes on the wall. In the above image, the stripes are larger and brighter at places where the waves join. The gaps between the stripes are the result of destructive interference, and the stripes are the result of constructive interference.
The photoelectric effect
The above experiment shows light behaving as a wave. But Albert Einstein showed us we can also describe light as being made up of individual particles of energy: photons. This is necessary to account for something called the “photoelectric effect”.
When you shoot light at a sheet of metal, the metal emits electrons: particles that are electrically charged. This is the photoelectric effect.
Prior to Einstein, scientists tried to explain the photoelectric effect by assuming light only takes the form of a wave. To understand their reasoning, imagine ripples in a pond. The ripples have peaks where the wave rises up, and troughs where it dips down.
Read more: Curious Kids: how do ripples form and why do they spread out across the water?
Now imagine there’s also a boat in the pond with Lego soldiers aboard. As the ripples reach the boat, they have the potential to throw the soldiers off. The more energy the ripples carry, the greater the force with which the soldiers will be thrown off.
And since each ripple can potentially throw off a soldier, the more ripples that reach the boat within a certain time limit, the more soldiers we can expect will be thrown off during that time.
Light waves also have peaks and troughs and therefore ripple in a similar manner. In the wave theory of light, these oscillations are linked to two properties of light: intensity and frequency.
Simply put, the frequency of a light wave is the number of peaks that pass a point in space in a given period (like when a certain number of ripples strike the boat within a specific time). The intensity corresponds to the energy of the wave (like the energy carried by each ripple in our pond).
Scientists in the 19th century pictured electrons on a sheet of metal as behaving similarly to the Lego soldiers on our raft. When light strikes the metal, the ripples should throw the electrons off.
The greater the intensity (the energy of the ripples) the faster the electrons will fly off, they thought. The higher the frequency within a specific time period, the greater the number of electrons that will get thrown off during that time — right?
What we actually see is the complete opposite! It’s the frequency of the light hitting the metal which determines the speed of the electrons as they shoot off. Meanwhile the intensity of the light, or how much energy it carries, actually determines the number of electrons flying away.
Einstein’s explanation
Einstein had a great explanation for this peculiar observation. He hypothesised light is made of particles, and is in fact not a wave. He then linked the intensity of light to the number of photons in a beam, and the frequency of light to how much energy each photon carries.
When more photons are shot at the metal (greater intensity), there are more collisions between the photons and electrons, so a greater number of electrons are emitted. Thus, the intensity of the light determines the number of electrons emitted, rather than the speed with which they fly off.
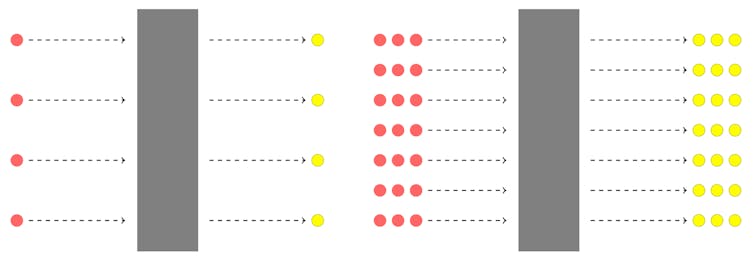 Increase the intensity of light, and therefore the number of photons bombarding a sheet of metal, and you’ll also see a greater number of electrons being shot off.
Provided by author
Increase the intensity of light, and therefore the number of photons bombarding a sheet of metal, and you’ll also see a greater number of electrons being shot off.
Provided by author
When light’s frequency is increased and each photon carries more energy, then each electron also takes more energy from the collision — and will therefore fly off with more speed.
This explanation earned Einstein a Nobel Prize in 1921.
Wave or particle?
Considering all of the above, one question remains: is light a wave that sometimes looks like a particle, or a particle that sometimes looks like a wave? There is disagreement about this.
My money is on light being a wave that displays particle-like properties under certain conditions. But this remains a controversial issue — one that takes us into the exciting realm of quantum mechanics. I encourage you to dig deeper and make up your own mind!
Read more: Curious Kids: Why is the sky blue and where does it start?
Authors: Sam Baron, Associate professor, Australian Catholic University
Read more https://theconversation.com/curious-kids-is-light-a-wave-or-a-particle-162514






